CHEMISTRY: Groundwater Risks from Venting R-1234yf
This document provides basic background on HFO/PFAS components to enable the protection of the U.S. water supply. 1234yf has been introduced by HFC Coalition members into patented blends in recent years, which means it will be required in order to reclaim those blends, but which presents significant environmental impacts. Those environmental impacts are likely to lead to future restrictions that in turn further complicate or prevent the reclamation of blends that contain this component. The result of this dynamic is that virgin producers effectively transfer the cost burden of future refrigerant management onto reclaimers and society more generally.
R-1234yf transforms into TFF (CF3COF) upon reacting with water in the atmosphere, ultimately leading to the formation of TFA. The widespread contamination of water supplies globally by TFA has raised concerns. In contrast, R-1234ze(E) exhibits a significantly shorter atmospheric lifetime of approximately 4 days and does not manifest similar behavior. It is crucial to emphasize that all HFOs are not uniform in their characteristics. Our primary objective is to enlighten stakeholders about the profound consequences of obstructing the reclamation of products containing 1234yf. A meager 1.6% reclaim rate for such products could result in catastrophic environmental impacts for municipalities and cities. While coalition members with long-term chemical-producing assets may dispute this, we underscore the potential trajectory of these products, such as refrigerant R454B, entering the water supply by elucidating the underlying chemistry.
In the atmosphere there are two decomposition pathways that R-1234yf undergoes. The first pathway is the reaction with hydroxyl radicals (-OH), which produces a 100% yield of trifluoroacetyl fluoride (CF3COF, TFF) while reaction with chlorine radicals produces a 92% yield of TFF:
CH2=CFCF3 + -OH → CH3COF + H2O + XO2 (1)
CH2=CFCF3 + Cl- → 0.92 CF3COF + 0.568 HC(O)Cl + XO2 + CO (2)
The products (intermediates) of pathway (1) are TFF and formaldehyde (HCHO) and pathway
(2) TFF and formyl chloride (HC(O)Cl). TFF then reacts rapidly with atmospheric moisture (H2O) to form trifluoroacetic trifluoroacetic acid (CF3COOH, TFA):
CF3COF + H2O → CF3COOH + CO2 + HF (3)
R-1234yf has four fluorine atoms on one side of the double bond, where the structure is CH2=CFCF3. The attack is thus:
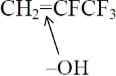
The result is that all four fluorine atoms are present in the decomposition product CF3COF (TFF), representing the decomposition product from the right side of the double bond. For 1234ze(E), one fluorine is on the left side of the double bond and three fluorines on the right side of the double bond. The attack is thus:
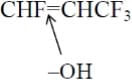
The breakdown of 1234yf yields two oxygenated byproducts, HCOF and CF3COH, a result of the molecule’s cleavage at its double bond. It is noteworthy that the once-unknown repercussions of 1234yf are now recognized.
This specific example is shown to demonstrate the historical and future impact of patented refrigerants touted as being the “cure” to refrigerant environmental issues. R11 and R12 were created to solve flammability when compared to hydrocarbons (R290, R600a) but resulted in an unintended consequence of creating a hole in the ozone layer. It is crucial to note that the same HFC coalition members amassed considerable profits during the shift from hydrocarbon refrigerants to CFCs, accumulating billions. As the patents for CFCs expired, these companies continued to reap substantial profits by transitioning to patented HCFCs like R22, contributing to a reduction in ozone layer depletion. Following the expiration of these patents, the same companies accumulated substantial wealth through the use of HFCs, including patented variations that contributed to global warming, while impeding reclaim. The evolution of subsequent generations of products still involves patented versions of HFCs with HFO additives like 1234yf. When 1234yf loses its patent protection, a new PFAS phasedown is anticipated, impacting the U.S. population and restricting access for reclaimers to 1234yf while introducing the next molecule to address the previously created problem. In consideration of these environmental windfalls, it is imperative that these companies, which have reaped significant profits, actively endorse unencumbered reclaim initiatives and life cycle management responsibilities to rectify the pollution they unleashed and prevent its entry into the U.S. water supply.
David L. Couchot
President & CEO
FluoroFusion Specialty Chemicals, Inc.

